A clinical data warehouse is a
collection of data that is originated from an operational database management system.
The purpose of this is for analysis and reporting of clinical trials data for the
FDA, usually in the form of an electronic submission. The clinical data warehouse is
organized by subject such as projects and studies. It is different from an
operational database in that it may contain summarized data for a particular
analysis.
The Trialex System is a web-based
system that automates the creation and maintenance of a clinical data warehouse. It
creates an environment to manage:
- Reports
- Source Data
- Analysis Data
- SAS Programs
- Dependencies
- Status
- Documentation
The Trialex System enables you to
capture and manage the metadata pertaining to these objects within the clinical data
warehouse. It sets standards by organizing the objects in an object-oriented model.
It manages the dependencies between the objects and the refreshing of the warehouse
by submitting the programs in their proper order. The attributes of all the objects,
from titles to reports, are centrally managed and easily updated through a friendly web
interface. The Trialex System automates the management of status among the objects
and facilitates the communication among team members. It compiles all the
information into a concise and accessible documentation.
The focus of the clinical
warehouse is on the end results which are usually reports. The flow leads up to the
reports as follows:
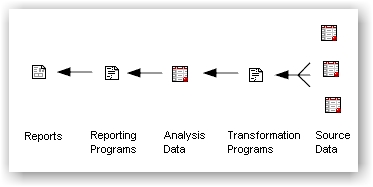
The clinical data warehouse is a
collection of many objects similar to the ones shown in the diagram.
The Trialex System requires SAS
6.12 on the server machine running Windows NT or UNIX. The client's machine uses a
web browser IE, or Netscape 4.0 or greater, to access the Trialex System. An email
client is also required for the client's machine.
The following lists the main pages
of the Trialex System. Each page is followed by a brief description.
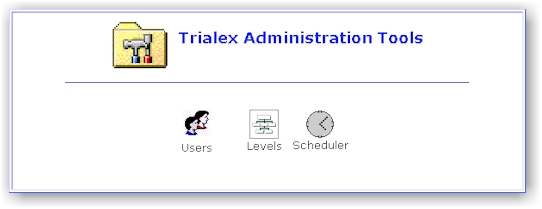
Administration Tools
- The administrator's main page. This contains tools to set up users,
clinical levels, job schedules.

Study Design - This
contains design tools including object dependencies and level designs.

Study Conduct - This
page contains all the tools to carry out the tasks of a clinical study. This
includes the management of the objects, reports, programs, data. This also includes
tasks such as refreshing objects, first pages, table of contents, status and
documentation.
The Trialex System can be
customized to fit the work environment. It is recommended that this activity be
reserved for an administrator so that changes occur consistently. There are some
graphical tools which assist in configuring the Trialex System but, in general, this is
done in batch mode by a tool named %config. This section will introduce some of the
configuration settings and is not intended to be a complete reference. For more
details, please refer to the configuration reference
section.
Before studies are set up in the
Trialex System, the organizational hierarchy, in which the studies are defined, needs to
be established. This is found in the levels section in the administration tools
area.

Levels defined in this section
will affect how standards are established since the studies will be defined within each
level. More detailed description of the level management tools are available in the reference section.
Many of the pull down menu items
within the Trialex System are customizable. This is usually established during
installation although changes can be applied at any time. One example is the report
type. This categories the different types of reports for easier access. The
report type is shown in the pull down menu of the report object as shown here:
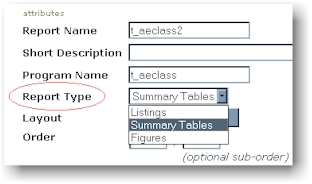
The report type can be used in
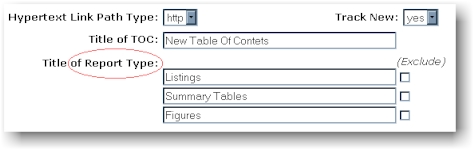
multiple areas. Another example where the report type is referenced is in the
options for generating the Table of Contents (TOC). In this example, the report
types are choices which can be excluded form the TOC.
The values which define the report
type can be configured so that all references to it will contain the same list. This
is defined with a batch macro named %config. This macro has to be invoked for each
report type. In the example above, the SAS program utilizing the %config macro would look
like:
%config (param= Report Type [1], value=Listings);
%config (param=Report Type [2], value=Summary
Tables);
%config (param=Report Type [3], value=Figures);
For central management of these
types of customization settings, it is recommended that all the values defined, such as
the report type, be included in one centralized SAS program. This way, it is easy to
find all the settings in one central location. This is one example of many
parameters which can be configured. For more details on the other options, refer to
the administration configuration section.
|