|
What is Symap MedDRA?
Symap Meddra is a software application designed for the mapping of adverse events. Adverse events are collected as part of a clinical trial and are mapped to industry standard preferred names. The mappings are implemented with the
MSSO MedDRA dictionary.
Symap allows Clinical Data Managers to map adverse events to preferred names, while continually updating the global dictionary and its derivative project-specific dictionaries.
An external source dictionary, MSSO MedDRA, is used as a source dictionary. The external dictionary is updated by
Symap only when new terms are added by the authors of the external dictionary.
Clinical Data Managers, for example, can map the preferred
adverse event name "Headache NOS" to the word "ache
head."
The mapping process will manually or automatically update the global dictionary and its derivative project-specific dictionaries to reflect the new mapping. Once updated, the dictionaries are applied to a specified source dataset.
In this way a new analysis dataset is created.
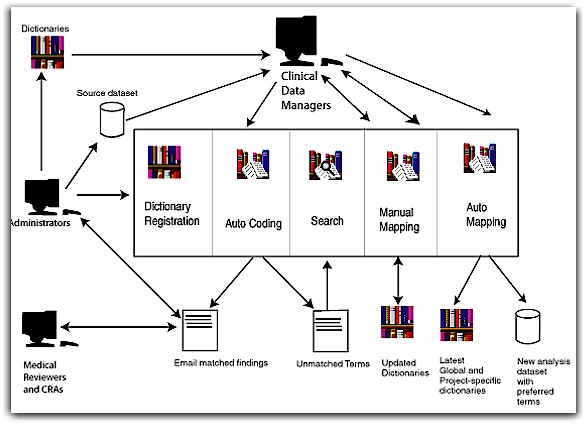
How it
Works
Following is a description of the Symap process flow.
Administrators of Symap register dictionaries into
Symap. There are three types of dictionaries that may be registered in any combination: project or site-specific, global (containing all
installed site specific terms), and external (a source dictionary updated by the system only when new terms are added by the
authors).
Clinical Data Managers use the Symap auto-coding feature in which unmapped adverse events contained in a specified source dataset are mapped to names contained in associated dictionaries.
Symap produces a listing of matched and unmatched terms for review.
Matched terms are sent to Medical Reviewers and Clinical Research Associates (CRAs) through
email for confirmation.
Clinical Data Managers conduct dictionary searches
on unmatched terms.
Clinical Data Managers manually map all unmatched terms.
Each term is mapped individually and if final mapping is designated, all registered dictionaries, except the external one, are updated to reflect each mapping decision.
Note that Clinical Data Managers may specify a tentative mapping in which the term is held for review and the dictionaries are not updated.
After the manual mapping is completed, Clinical Data Managers use
"mapex" (an automatic mapping process) to associate the adverse events in the source data with preferred names in the specified dictionaries, resulting in a new
"mapped" analysis dataset.
When the mapex process is completed and the destination dictionary defined for the mapping session is updated with the matched information, all dictionaries (except external) are updated with all previous mapping decisions.
Following is a diagram of the mapping process.
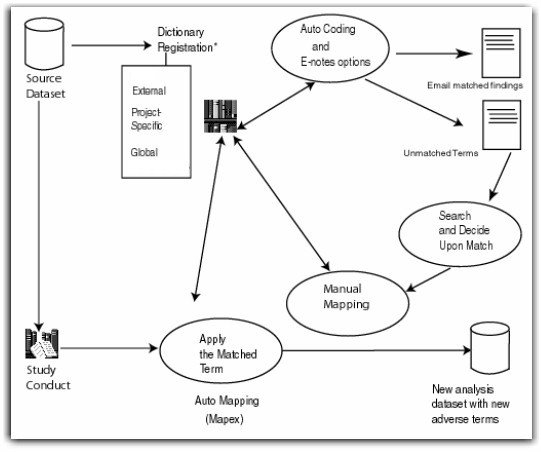
|